The Hidden Cost of Surgical Instrument Tracking in a $400 Billion Industry
The global medical device industry is valued at over $400 billion, yet one of its most fundamental operational challenges remains stubbornly unsolved: tracking individual surgical instruments accurately and efficiently. Behind every surgical procedure lies a complex web of inventory, logistics, consignment agreements, compliance requirements, and human processes—many of which are still manual, error-prone, and opaque.
The result is not just wasted money. It’s lost time, strained relationships between hospitals and distributors, compromised surgeon preferences, and, most critically, real downstream impacts on patient morbidity and outcomes.
iTRACE 2DMI: A Foundation for Surgical Intelligence
iTRACE addresses these systemic challenges by uniquely identifying every individual surgical instrument—not just trays or sets, but each tool itself.
This shift from batch-level to item-level intelligence unlocks transformative advantages across the surgical ecosystem.
How iTRACE 2DMI Changes the Equation
1. Increased Efficiency at Every Stage
From sterilization to storage to the OR and back, instruments are identified instantly—eliminating manual counts and guesswork.
2. Improved Patient Outcomes
Accurate instrument availability reduces delays, errors, and substitutions, directly contributing to lower morbidity and mortality rates.
3. Surgeon Preference Optimization
Surgeons consistently receive the exact instruments they prefer, improving confidence, performance, and outcomes.
4. Fast Identification of Missing Instruments
Lost or misplaced tools are identified immediately, reducing OR delays and frantic post-case searches.
5. Reduced Loss and Unplanned Replacement
Knowing where each instrument is—and its true lifecycle—prevents premature write-offs and unnecessary reorders.
6. Lower Inventory Costs and Overhead
Hospitals and distributors can safely reduce excess inventory while maintaining availability.
7. Accurate Provenance and Circularity
Every instrument’s history is traceable: usage count, location, sterilization cycles, and authenticity. Fully lifecycle management is delivered with actionable data and insights.
8. Faster Reporting, Fewer Manhours
Automated data capture dramatically reduces time spent on audits, updates, and charge reconciliation.
9. Happier Reps and Better CSP Setups
Sales and distributor reps spend less time counting and reconciling—and more time supporting surgeons and value-based care models.

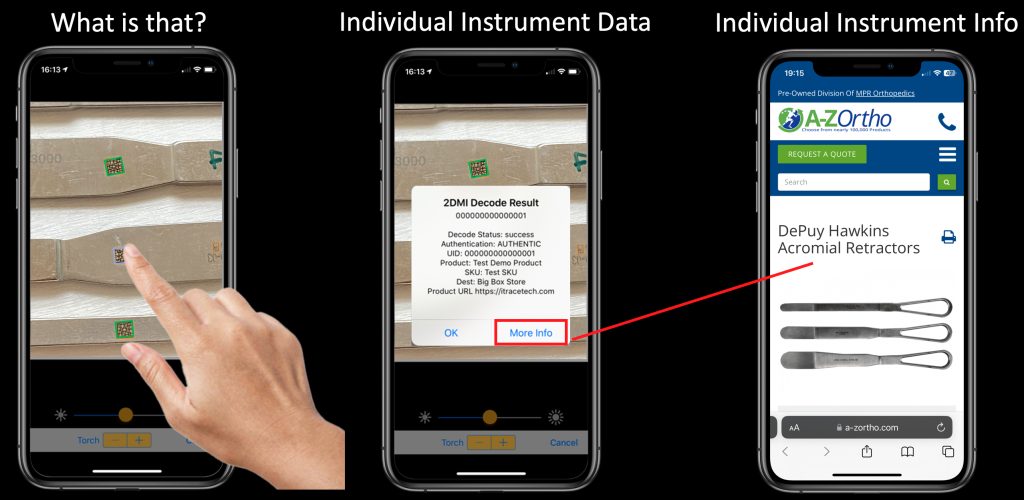
A Simple Mobile App Scan Enables Full Instrument Provenance
A simple mobile scan can identify a complete tray of instruments allowing every point in the supply chain to participate in the tracking and provenance of each instrument. Each instrument can be identified individually and data about that instrument provided to every user.
Patient Safety at Stake: When Instrument Tracking Fails
Beyond financial waste and operational drag, the most serious consequence of poor surgical instrument tracking is patient harm. Retained Surgical Instruments (RSIs)—sometimes referred to as retained surgical bodies—are among the most preventable yet devastating “never events” in healthcare.
And they are not rare.
Real-World Horror Stories
Recent high-profile cases underscore just how catastrophic these failures can be:
- A plate-sized surgical tool was left inside a woman’s abdomen for 18 months, causing severe pain, infection, and long-term complications before it was discovered.
- Another woman endured years of suffering while fighting to identify the doctors responsible for forceps left inside her body after surgery.
These cases are not isolated anomalies—they are symptoms of systemic tracking failures in environments that still rely heavily on manual counts, visual checks, and human memory under extreme pressure.
The Numbers Are Alarming
In the United States alone:
- The rate of retained surgical bodies ranges from 0.3 to 1.0 per 1,000 abdominal surgeries
- The most common locations include:
- Abdomen
- Pelvis
- Retroperitoneum
There are approximately 105 million major surgeries performed in the U.S. each year.
Even at the low end of reported rates, this translates to tens of thousands of retained surgical items annually. At higher estimates, the number approaches over 100,000 retained surgical bodies every year.
And these figures typically focus on large, countable instruments.
The Uncomfortable Question: What About Smaller Items?
What often goes unreported—and undercounted—are smaller surgical components:
- Broken instrument tips
- Detachable parts
- Worn or fractured tools
- Non-radiopaque items
- Instruments substituted mid-procedure
These items are far harder to track, easier to miss during manual counts, and more likely to evade post-operative detection until complications arise. In many cases, patients only learn something is wrong months—or years—later, after infections, pain, or repeat surgeries.
You Can’t Do This With Standard Barcodes, RFID or NFC
Many technologies have been tried in pursuit of individual instrument tracking including all types of 2D codes, RFID and NFC. None have been able to deliver on the promise. iTRACE 2DMI is proven to deliver secure track and trace with simple mobile app scans. iTRACE enhanced computer vision applications can provide a reliable, highly damage resistant mobile application for surgical instrument tracking.

